
Fcn Lewis Dot Structure Abbreviations Fch Family Cohesion Fcn Family
FCN Lewis Structure FCN is a chemical formula for cyanogen flouride. And to help you understand the Lewis Structure of this molecule, we are going to.

How To Draw Lewis Structures In Chemdraw Images and Photos finder
6 Steps to Draw the Lewis Structure of FCN Step #1: Calculate the total number of valence electrons. Here, the given molecule is FCN. In order to draw the lewis structure of FCN, first of all you have to find the total number of valence electrons present in the FCN molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
Draw Lewis Structure
A step-by-step explanation of how to draw the CN- Lewis Dot Structure (Cyanide ion).Note: there should be brackets around the final Lewis Structure with a -1.

[DIAGRAM] Kcl Lewis Dot Diagram
FCN is a chemical formula for cyanogen fluoride. And to help you understand the Lewis Structure of this molecule, we are going to share our step-by-step met.

FCN Lewis Structure How to Draw the Lewis Dot Structure for FCN
Choose the best Lewis structure for FCN and explain why it is the best C==N: 6_C=n: Structure is better because it is symmetrical with 2 double bonds Structure b is better because it has all formal charges that are zero while structure has a + | charge on the N and a - ] charge on the F

Breathtaking Tips About How To Draw A Lewis Dot Structure Ballchicken
FCN lewis structure, also known by the chemical name as cyanogen fluoride, is an inorganic molecule with a molecular weight of 45.016 g/mol. Some facts about FCN lewis structure : Molar mass/Molecular weight = 45.016 g/mol. Boiling point = -46.17 0 C , Melting point = - 82 0 C .
Draw Lewis Structure
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
lewis dot structure chart Focus
About. Transcript. We can draw Lewis structures for polyatomic ions (ions containing multiple atoms) using the same stepwise procedure as for neutral molecules. In this video, we'll see how to construct the Lewis diagram of the cyanide ion (CN⁻). Created by Sal Khan.
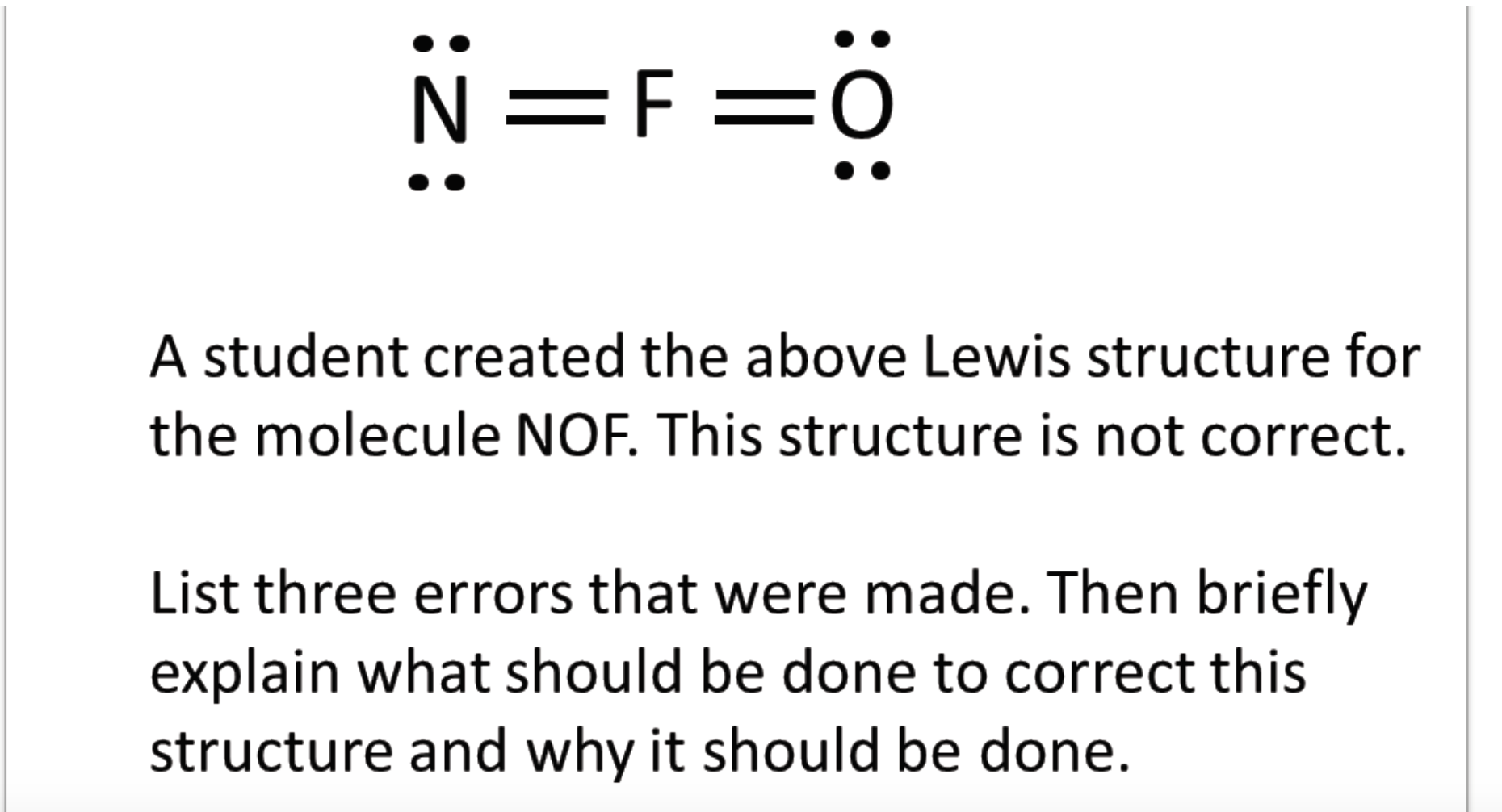
Solved N=F =0 A student created the above Lewis structure
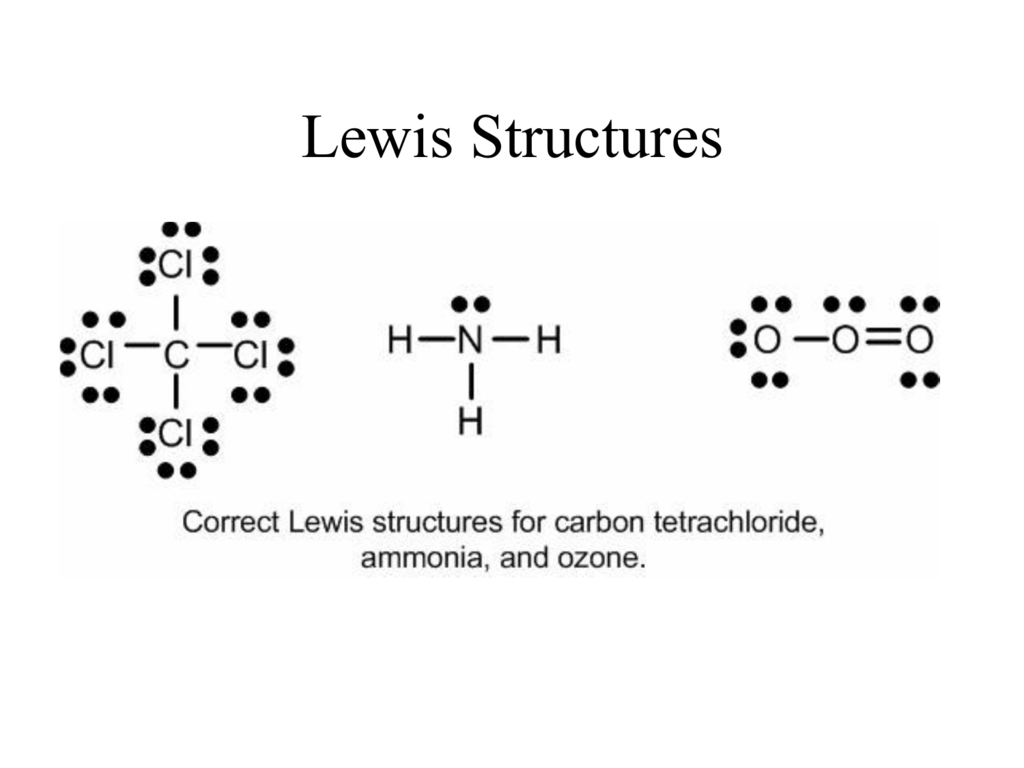
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)
Lewis Structure Definition and Example
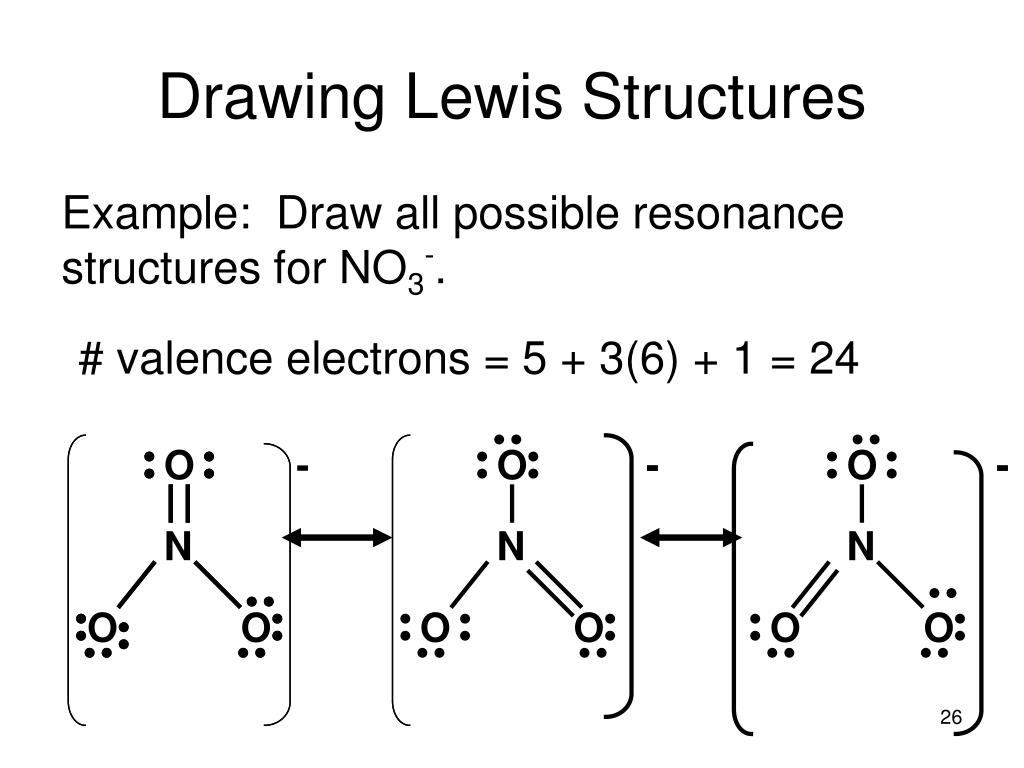
8.6: Resonance Structures is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by LibreTexts. Some molecules have two or more chemically equivalent Lewis electron structures, called resonance structures. Resonance is a mental exercise and method within the Valence Bond Theory of bonding that..

C2h2br2 Nonpolar Lewis Structure
What is the Lewis structure of [//substance:FCN//]? Natural Language; Math Input; Extended Keyboard Examples Upload Random. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports.
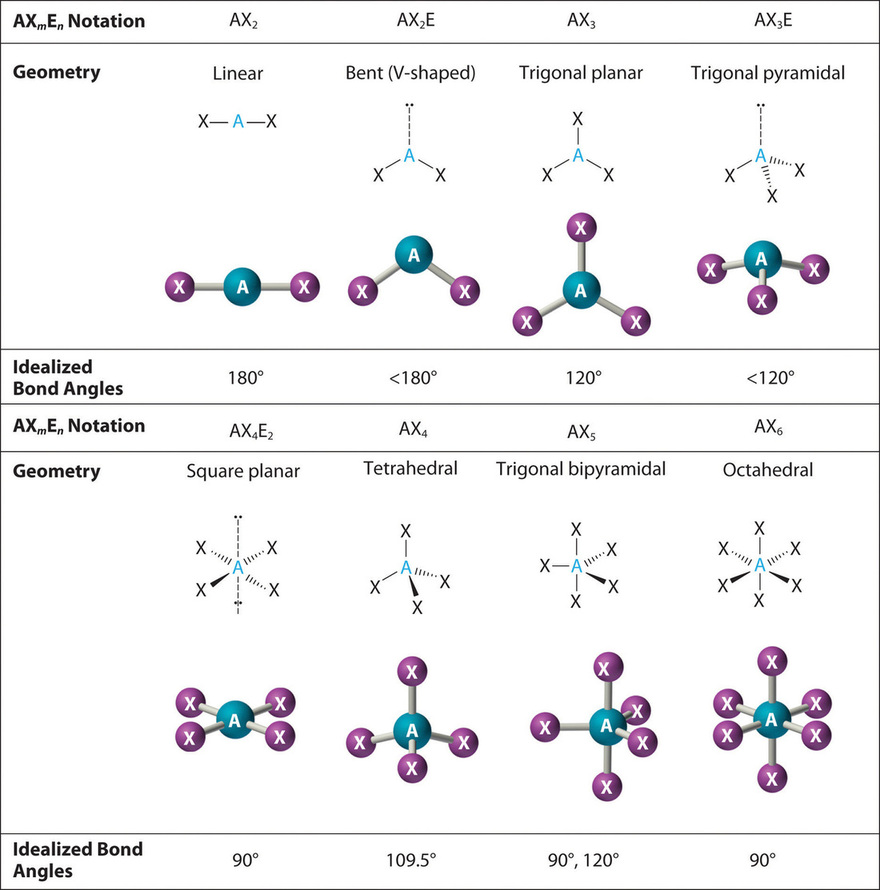
The VSEPR Model
Summary. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. There are three exceptions: (1) When there are an odd number of valence electrons, (2) When there are too few.
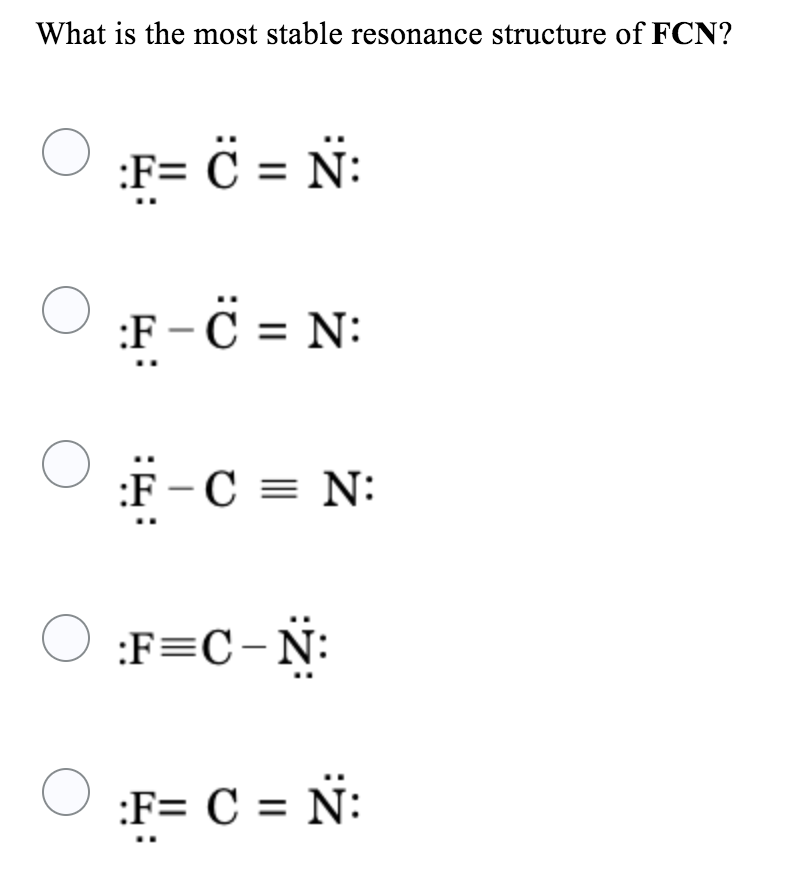
Solved What is the most stable resonance structure of FCN?
Cyanogen fluoride (molecular formula: FCN; IUPAC name: carbononitridic fluoride) is an inorganic linear compound which consists of a fluorine in a single bond with carbon, and a nitrogen in a triple bond with carbon. It is a toxic and explosive gas at room temperature. It is used in organic synthesis and can be produced by pyrolysis of cyanuric fluoride or by fluorination of cyanogen.
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
Best How To Draw The Lewis Dot Structure in the year 2023 The ultimate
A step-by-step explanation of how to draw the FCN Lewis Dot Structure.For the FCN structure use the periodic table to find the total number of valence electr.

How to Draw Lewis Structures RodneytaroBall
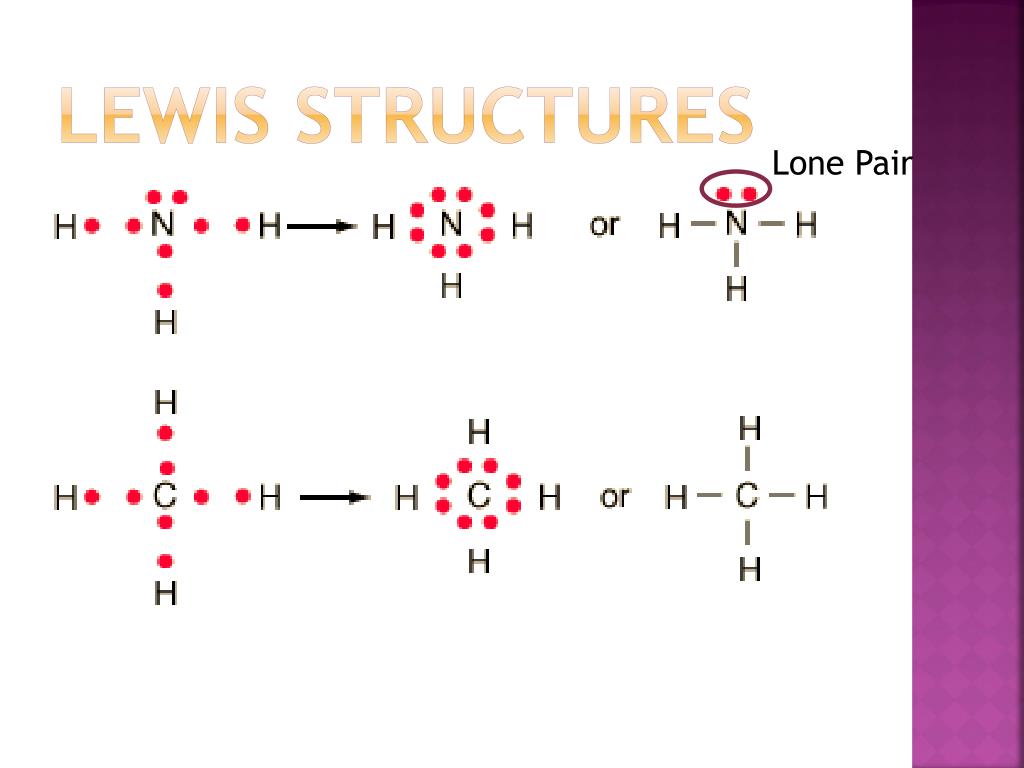
The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well.

Draw Lewis Structure
In FCN Lewis structure, there is a single bond between carbon and fluorine atom, and a triple bond between carbon and nitrogen atom. The fluorine atom has three lone pairs, and the nitrogen atom has one lone pair. Contents. Steps #1 First draw a rough sketch